The Ambiguity of Reduction
Eric R. Scerri
Abstract: I claim that the question of whether chemistry is reduced to quantum mechanics is more ambiguous and multi-faceted than generally supposed. For example, chemistry appears to be both reduced and not reduced at the same time depending on the perspective that one adopts. Similarly, I argue that some conceptual issues in quantum mechanics are ambiguous and can only be laid to rest by embracing paradox and ambiguity rather than regarding them as obstacles to be overcome. Recent work in the reduction of chemistry is also reviewed, including discussions of the ontological reduction of chemistry and the question of the emergence of chemistry from physics.
Keywords: quantum mechanics, quantum chemistry, epistemological reduction, ontological reduction, emergence, positivism.
1. Introduction
When people ask me what topic my Ph.D. thesis was concerned with I respond, "On the question of whether chemistry is reduced to physics". This obviously invites the further question of "Well what did you conclude? Is chemistry reduced to physics or not?"
I have come into the habit of answering by saying "Yes and No". This is partly a joke and, partly, because I believe it is the best answer one can give to the question. In the present article I want to take seriously the ambiguities encountered in the discussion of reduction of the special sciences such as chemistry.
The response of "Yes and No" which I gave to the question above
can be taken in a weak sense to imply that chemistry has only been partly
reduced and this is indeed part of my message (Scerri 1994). However, the
response may also be taken to mean that chemistry is both reduced and not
reduced at the same time. It is the latter apparently ambiguous response that I
wish to pursue in the remainder of this article. Some of this ambiguity may be
due to a conflation of the questions of the epistemological and ontological
reduction of chemistry. Recent developments in the literature will be reviewed
in an attempt to clarify these issues. Finally some comments will be made on
the emergence or otherwise of chemistry from physics.
2. Quantum Mechanics and Quantum Chemistry
The development of quantum mechanics and its early successes, in explaining the bonding in the hydrogen molecule for example, led Dirac to make his much quoted remark that in principle the whole of chemistry had been explained by quantum mechanics (Dirac 1929). Quantum mechanics has continued to uphold such claims, while often conceding that the kind of reductive vision held by Dirac is somewhat untenable. The chemical community has been partly forced into accepting the reductive claim because there is no denying that the quantitative approach affected via quantum chemistry has made a considerable contribution to chemistry in a variety of different areas. Computational packages are now a commonplace experimental tool with which any organic chemist can make fairly reliable predictions even if he or she does not possess the slightest knowledge of quantum mechanics or theoretical chemistry.
However, there have often been chemists with a philosophical bent who have resisted the reductive claims. The rapid growth of philosophy of chemistry which has been taking place since 1994 has made these objections more visible. Part of the motivation for these objections seem to have come from general philosophy of science and the widespread rejection of logical positivism with its associated position on the achievability of reduction. At the same time, it appears that some philosophers may have been a little too eager to abandon the viability of the reduction of chemistry, when one considers what working scientists believe to be the case (Gell-Mann 1994, Wilson 1989).
Some philosophers of chemistry, like van Brakel, have even been prepared to
argue against micro-reduction in general (van Brakel 2000). Interestingly the
only authors within the history and philosophy of science who regard the
reduction of chemistry to be largely unproblematic have been historians of
science (Bensaude & Stengers 1996, pp. 207-252; Knight 1992, pp. 157-170).[1] But there are at least two further complications that contribute
to what I am calling the ambiguity of reduction. These will be dealt with in
the following two sections.
3. The Changing Meaning of Reduction
Traditionally reduction has been associated with positivism and with the goal of anchoring scientific knowledge in macroscopically observable aspects of the world. One of the major arenas where this issue has been played out has been atomic theory. Positivists typically denied the existence of physical atoms, largely because they could not be observed. Atomic theory was regarded as anti-reductionist since the basic entities – atoms – could not be grounded in observation (Dumas 1837, p. 314). But starting with the work of Perrin and Einstein and culminating in the STM images of atoms that became available at the end of the twentieth century, the physical existence of atoms has become increasingly compelling. Reductionism in the old sense of anchoring theories to macroscopic observables has thus become redundant in the case of atoms since it is claimed that atoms can now be seen.
Instead, the term reductionism changed its meaning in such a way as to remain as a viable philosophy. Reductionism came to mean the attempt to explain macroscopic phenomena through recourse to microscopic components. Rather strangely the meaning thus became the opposite to what it had been previously. According to the success of atomic theory one could now maintain a reductionist philosophy by insisting that success had been gained by accepting microscopic explanations for the behavior of macroscopic matter.
This none too subtle shift in the meaning of the term reduction has been commented upon by Milton Rothman (Rothman 1992):
A peculiar turnabout occurred during the course of the twentieth century. Through the work of the physicists, atomic theory became so well established that it was no longer possible to attack it by calling it antireductionist. Philosophers had to reverse the position of reductionism in order to make atomic theory part of the establishment. Reductionism now embraced the idea that everything was made of atoms. As a result, the concept of reductionism now became the target of criticism from those who could not accept the idea that human beings were made of atoms and followed physical principles like everything else in nature.
Furthermore, since there are no longer any clear criteria for the establishment of reduction, as there might have been in logical positivist times, it is not surprising that opinions vary so widely between philosophers and scientists and even among authors working in the history and philosophy of science. One cannot help wondering whether the knee-jerk denial of reduction, which one encounters among some philosophers of science, is concerned with the old sense of reduction and the associations with the demise of positivism. The few exceptions among philosophers who are willing to discuss reduction in the sense of reduction to smaller components, and to the basic laws of physics, have included Popper (1974) and Suppes (1984).
Some philosophers of chemistry, including Schummer, Psarros, and van Brakel, have presented a somewhat radical opposition to the reductive claims (Schummer 1997, Psarros 1997, van Brakel 2000). These authors do not dispute the details of the quantum mechanical treatment of chemical bonding or explanations of the periodic system etc. Instead, they have independently concentrated on pointing out the undeniable fact that chemistry is primarily the science of substances. Their view is essentially that any micro-reductive account provided by quantum mechanics fails to give an adequate description of substances in the form which is of interest to most chemists and perhaps even philosophers. For example van Brakel (2000, p. v) writes:
With respect to chemistry the question can be raised: where does it fit in – with the manifest or the scientific image? Most philosophers and chemists probably would reply unhesitatingly: the scientific image. The aim of this book is to raise doubts about that self-evidence. It is argued that chemistry is primarily the science of manifest substances, whereas ‘micro’ or ‘submicro’ scientific talk, though important, useful, and insightful does not change what matters, namely the properties of manifest substances. These manifest substances, their properties and uses cannot be reduced to talk of molecules or solutions of Schrödinger’s equation. If ‘submicroscopic’ quantum mechanics were to be wrong, it would not affect all (or any) ‘microlevel’ chemical knowledge of molecules. If molecular chemistry were to be wrong, it wouldn’t disqualify knowledge of, say, water – not at the macrolevel (e.g. its’ viscosity at 50°C), nor at the pre- or protoscientific manifest level (e.g. ice is frozen water).
This is of course quite correct and van Brakel is to be applauded for putting the case so cogently. Other authors have made more specific critiques of the reduction of chemistry where the aim is not to deny the whole enterprise but to discuss certain aspects of it (Hendry 1998, Ramsey 1997, Scerri 1998, Woolley 1985, Weininger 1984, Woody 2000).
In my own work on the periodic system I have claimed that reduction of the
periodic law to quantum mechanics is not complete (Scerri 1998a). But in doing
so I attempt to provide an analysis of what is wrong with the present reductive
claim with the view that it might be possible to achieve an even deeper level
of reduction. My intention is not to deny the viability of the reductive
program but to clarify the claims and to examine what has been achieved up to
the present time (Scerri 1999). In this work I do not deny that the
epistemological reduction of chemistry could be achieved but only that this has
not been the case up to now. I think that this position is rather far removed
from the more outright rejection of the reductive program as favored by some
philosophers that I have mentioned above.
5. Ambiguous Aspects of Quantum Physics and the Reduction of Chemistry
It is proposed that the notion of ambiguity should be positively embraced in
physics and in the question of the reduction of chemistry. Although I am
primarily interested in the reduction of chemistry to quantum mechanics, I will
also consider the element of ambiguity in quantum physics. I take this approach
in view of the close connection between quantum physics and chemistry,
particularly in the context of reduction as understood in naturalistic terms
rather than the traditional view involving axiomatization (Scerri 1998). The
view that some philosophers now favor is that the epistemological reduction of
chemistry is best considered as a relationship between chemical properties and
the equations of quantum mechanics rather than as a formal logical relation
between axiomatized theories of chemistry and quantum mechanics.[2]
One of the problems of the traditional view is the fact that chemical theories
do not lend themselves to axiomatization. I have previously suggested that the
reduction of chemistry as understood by scientists is more a question of
whether chemical phenomena can be deduced from the first principles of the
reducing theory or quantum mechanics in this case (Scerri 1994).
6. Ambiguity in Quantum Physics
Let me begin with a familiar example that I will base on the presentation in a chemistry textbook intended for physical science majors. In discussing wave-particle duality the author, Michael Munowitz, begins by explaining the notion of diffraction and interference of water waves (Munowitz 2000).
Munowitz then reviews a familiar episode in the history of quantum mechanics. It is well known that, in trying to explain the photoelectric effect, Einstein argued that light has particulate as well as wave-like nature. This already represented one level of ambiguity which quantum physicists were forced to accept. De Broglie then suggested that electrons might possess wave-like character. The intuition to further embrace wave-particle duality, but in the other direction, also turned out to be very fruitful since it was soon confirmed that electrons did indeed show wave-like behavior. These developments then led to the discovery of wave mechanics at the hands of Schrödinger and others.
The ambiguity, some might say paradox, lies in the fact that the particle nature and wave nature appear to be diametrically opposed aspects of reality.[3] Whereas particles represent the epitome of localized objects, waves are infinitely extended in space. How could two such apparently different features of reality be so intimately related? And yet waves and particles must be regarded as compatible, or perhaps fundamentally identical in nature, in trying to understand the behavior of both waves and particles. What were formerly regarded separately as waves (electromagnetic radiation) or particles (electrons etc.), each of which give rise to diffraction and interference effects, must now be viewed as being a little of both. Waves are as much particles as they are waves and particles are as much waves as they are particles.
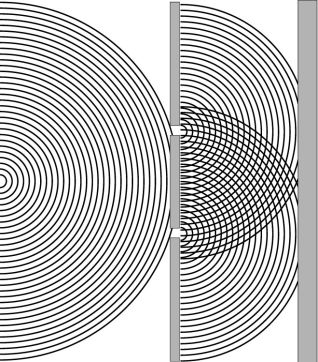
Figure 1. Diffraction and interference of water waves.
Munowitz proceeds to give the reader a taste for the conceptual problems of modern physics by revisiting the well-known double-slit experiment. Like so many authors before him he asks the question of why it is that the interference pattern is observed even if the stream of electrons hitting the two-slit arrangement is slowed down to a mere trickle. Does the electron passing through one particular slit somehow ‘know’ that the other slit is open? Surely the electron cannot spit into two particles writes Munowitz. After all, he might have added, the electron is still considered as the archetypal structure-less particle, which cannot be sub-divided.[4]
In providing this kind of presentation it strikes me that Munowitz, in common with many other authors, is in fact retreating from wave-particle duality. He is retreating from the full acceptance of the ambiguity which quantum physics demands of us. After spending several pages in trying to convince the reader of the validity of wave-particle duality he does not seem to want to truly ‘bite the bullet’ of duality.
If the electron really does have a wave-like character, then why not regard it as giving rise to a wave front, albeit in three dimensions, by analogy to the water waves in Figure 1? If this is assumed to be the case, the conceptual worries about the two-slit experiment are at least alleviated. The electron, now seen as a wave, can indeed be considered to have split into two parts, which then interfere to produce the familiar pattern of dark and bright fringes in the image observed at the screen.
Provided that we are willing to embrace the ambiguity that the electron
displays wave-like character, there is no problem in reconciling the observed
facts. Even a slow stream of electrons, passing ostensibly through just one
slit, will gradually produce the interference pattern that would be expected if
electrons were passing through both slits. In fact, according to some
commentators, this is exactly how the Copenhagen approach to quantum mechanics
resolves the problem, namely by accepting the full implications of the
ambiguous nature of what were formerly regarded distinct phenomena, namely
waves and particles (Baggott 1992).[5] But the concern that
still remains is that this explanation seems to require a complete denial of
the particle nature of the electron that is not entirely satisfactory.
7. Where Does Chemistry Come in?
Now to return to chemistry, I am going to claim that the reduction of chemistry is ambiguous, in this same positive sense as the case of wave-particle duality. In the case of chemistry the ambiguity consists in the fact that the reduction of chemistry is both successful and unsuccessful depending on the perspective adopted.
It is successful, just like reductive approaches have been highly successful in other parts of science since the development of mathematical science at the hands of Galileo, Newton, Dalton, Maxwell, Boltzmann, and many others. The reductive approach consisting of the mathematical analysis of nature in terms of fundamental microscopic components has repeatedly shown itself capable of rationalizing and accommodating already known scientific phenomena as well as in making new predictions of previously unknown facts. In this respect the success of the reductive enterprise is undeniable even if not complete.
Whereas it used to be said that quantum chemistry could only reproduce already known facts, the situation has changed dramatically as a result of the rapid development in computational power. The fact that quantum chemistry deals in approximations rather than in analytical solutions is of little consequence given the power of modern methods of approximation.
The recent developments in quantum chemistry are somewhat analogous to the rapid developments that have occurred in weather forecasting and which can be traced to the same cause, namely enhanced computational power. Whereas it was always a standing joke that weather forecasts were invariably wrong, the same cannot be said of the current state of the art. The recent improvement is partly due to the rapid proliferation of satellite technology, which allows one to collect more data points. But it is especially due to the current ability to handle vast amounts of data and to carry out parallel computations of the complex mathematical equations that describe weather patterns. My point, regarding quantum chemistry as well as meteorology, is that the success of these fields is completely undeniable. Seen from this perspective reduction has been, or is well on the way to being, achieved.
But reduction of this kind has not been successful in chemistry in not providing a conceptual understanding of chemical phenomena. Given the essentially holistic nature of conceptual understanding, this is not altogether surprising of course. To return briefly to quantum mechanics as distinct from quantum chemistry, it is now common knowledge that the former theory is the most widely corroborated theory compared to any other theory ever devised. However, the conceptual issues revolving around wave-particle duality and the collapse of the wave function have continued unabated for eighty years or so since the contributions of Schrödinger, Heisenberg, and others.[6]
Similarly, the highly accurate quantum chemistry calculations are empirically very adequate and yet chemists continue to rely on classical explanatory models such as Lewis structures and VSEPR theory to name just two examples. The fact that they do so is also not surprising since these models and concepts operate at the appropriate chemical level which is familiar to chemists, namely the talk of pairs of electrons, atoms, and bonds. By turning to quantum mechanics the chemist can generate quantitative values for bond angles, bond strengths, or dipole moments, but such calculations are not a substitute for the classical explanatory schemes which continue to be used by chemists.
In addition chemists continue to rely on electronic configurations for atoms and molecules that cannot themselves be strictly predicted fully from quantum mechanics. For example, they use three principles to deduce the configuration of any atom. These are the aufbau, Hund, and Pauli principles, none of which have themselves been deduced from quantum mechanics (Scerri 1998a).
Equally important perhaps, the periodic table, the central classical explanatory
model of the chemist, has not been deduced from quantum mechanics. All that can
be done is to give a quantum mechanical justification for electronic
configurations that are obtained empirically from atomic spectra. One cannot
begin with quantum mechanics alone and predict the configuration of a
particular atom (Scerri 2004, 2007). On the other hand, given several possible
candidate configurations, quantum mechanics can successfully select the
configuration that corresponds to spectral observations on the system in
question. Quantum mechanical calculations can thus reproduce configurations but
cannot strictly deduce them from first principles, or at least this has not yet
been carried out.
8. Ontological Reduction and Emergence
All that has been said up to now has concerned epistemological reduction, namely the extent to which theories of chemistry have been or can be reduced to theories of physics. As Lombardi and La Barca (2005) have recently very cogently argued, the vast majority of authors working in the philosophy of science have assumed or have even stated explicitly that the ontological reduction of chemistry is completely unproblematic.[7] Lombardi and La Barca have opened up a new front by questioning and indeed denying the ontological reduction of chemistry. I take the ontological reduction of chemistry to physics to mean that chemical phenomena are fundamentally nothing but physical phenomena. This claim is made independently of any theoretical account of either chemistry or physics or any theoretical account of the connection between the two fields.[8]
On the other hand, there are authors who uphold the ontological reduction of chemistry. McLaughlin (1992) has given a detailed analysis of a number of authors whom he terms the British Emergentists, the leading representative of this group being C.D. Broad. Writing in the years immediately preceding the development of a quantum mechanical explanation of chemical bonding, Broad laid down some conditions under which emergence might be said to take place. For Broad emergence occurs if ‘downward causation’ is occurring. This in turn depends on the operation of what Broad calls ‘configurational forces’ which differ from resultant forces involving such fundamental interactions as the electromagnetic or gravitational forces. Broad’s main example of what he thought to be emergence was chemical bonding since, as McLaughlin explains, there was no adequate explanatory theory of chemical bonding at the time. By contrast McLaughlin claims that given the advent of quantum mechanics and the development of a quantum mechanical theory of bonding there is absolutely no room for the concept of downward causation via the operation of configurational forces. McLaughlin thus concludes that chemistry does not emerge from physics but that instead chemistry is ontologically reduced to physics.
More recently, the metaphysician Le Poidevin (2005) has published an article
in which he avoids all reference to theories of chemistry and physics in trying
to assess whether chemistry is reduced to physics in an ontological sense.
Among other things such an approach is supposed to avoid some of the problems
that plague physicalism.[9] Instead Le Poidevin opts for a ‘combinatorial
analysis’ with which he aims to explain why it was that Mendeleev was so
confident that the elements which he predicted were not mere logical
possibilities but also physical possibilities. As far as the present author is
concerned it seems as if Le Poidevin has indeed given a good argument for the
ontological reduction of chemistry at least as far as the existence of the
elements are concerned but not for the ontological reduction of chemistry tout
court.[10]
9. Some Preliminary Conclusions
Most discussions on the reduction of chemistry seem to conclude either in favor or, almost unanimously these days, against reduction of chemistry to quantum mechanics. Some authors who deny the reduction of chemistry fail to acknowledge the progress made in quantum chemistry and the quantitative success achieved by this field of chemical research. What they really mean in denying the reductive claims is that quantum chemistry fails to provide familiar qualitative, or model-based, explanations for chemical facts.
The reduction of chemistry is ambiguous in that it is both successful and unsuccessful depending on what one requires from quantum chemistry. This fact has not been generally accepted with the result that claims made in the philosophical literature against the reduction of chemistry are often over-stated (van Brakel 2000, Psarros 1997, Schummer 1997).[11] What is needed, in my view, is a dialogue between philosophers of chemistry to engage in dialogue with theoretical chemists just as philosophers of physics have maintained a dialogue with contemporary physicists.
The real challenge is to connect the purely reductive, mathematical, description of chemistry with conceptual models in a manner that can be readily assimilated by working chemists and chemical educators. It is not to dispute the success of the reductive approach as some contemporary philosophers of chemistry seem to want to do. The success of quantum chemistry as I have tried to emphasize is simply undeniable, at least in its quantitative sense. What is needed is a more critical examination of quantum chemistry rather than turning one’s back on the success of the field.
On the other hand the apparent success of quantum chemistry should not lead
one to believe that the ontological reduction of chemistry is a foregone
conclusion. The question of ontological reduction is more interesting
philosophically and one for which one needs to look beyond the success or
otherwise of quantum chemistry. As has been recently pointed out (Lombardi
& Labarca 2005), the generally assumed notion that chemistry does indeed
reduce to physics ontologically runs the risk of keeping philosophy of
chemistry as an area of marginal interest. In the section to follow I propose
to give a sketch of an argument for the emergence of chemistry, which provides
further support for the recent claim by Lombardi and LaBarca that chemistry
does not ontologically reduce to physics.
10. The Question of Emergence
Emergence has often been described as representing the opposite tendency to reduction. To be an emergentist is to hold that certain phenomena, forms of organization, etc., emerge at certain levels, over and above what one would expect from the constituents of the system.[12]
I will argue that from an evolutionary point of view it is necessary to acknowledge the occurrence of emergence and to deny the full effectiveness of reduction. To put the point in different terms, if one allows the element of time to enter into the picture, it becomes necessary to admit the occurrence of emergence. It will be argued that strict reductionism can only be maintained if one ignores the evolutionary or temporal dimension altogether.
One might consider the evolution of the universe as a whole. According to contemporary cosmological physics, there was the Big Bang and the subsequent formation of elementary particles such as electrons, protons, and neutrons. These particles then condensed into definite units to form atoms of the chemical elements, which then combined to form compounds and so on. It will be helpful to concentrate on one particular step in this evolutionary process, which is relevant to the chemistry/physics interface. For example, when five protons and five electrons combined together with the appropriate number of neutrons they formed atoms of boron. On the other hand when six protons and six electrons combined together with the appropriate number of neutrons they produced another element, namely carbon which is very different from boron, and which has turned out to be the basis of all living systems.
The point that I am driving at is that the mere addition of a proton, electron, and a few neutrons has brought about a profound and unpredictable modification in the properties of the atom of boron. New properties, in this case the ability to sustain living systems, appear to have emerged upon the addition of a few fundamental particles.[13]
But this emergence, or this qualitative change, as a result of moving from boron atoms to carbon atoms, takes on a far less impressive aspect if one denies the fact that carbon literally evolved from boron on the addition of further elementary particles. If one denies the element of time, or the evolutionary process through which these elements were formed, the reduction of the properties of the fully formed elements appears to be less problematic and there appears to be little or no scope for emergence of any kind. For example, the ionization energy for each of these atoms can be predicted to an extremely high accuracy from quantum mechanics, as can many other numerical properties of the atoms in question. But the fact that adding one proton and one electron to an atom of boron produces an element that is the basis of life is completely unexpected.
This feature incidentally introduces another aspect concerning the question of reduction. The numerical properties that are accessible to reduction via quantum mechanics tend to be distinct properties such as the energy of an atom, the bond angle of a molecule, and so on. This is not the same as being able to reduce the overall chemical behavior of atoms. For example, why is it that atoms of helium with two protons and two electrons are completely and utterly un-reactive while the addition of just one proton and one electron produces atoms of lithium, an element which forms a multitude of compounds and which reacts with water to form an alkaline solution? Similarly, in terms of macroscopic properties the two elements are also vastly different, since helium is a gas while lithium is a dull silvery metal.
The difference, which I have attributed to the presence or absence of time, may have its counterpart at a more fundamental level in theoretical physics. It is well-known that the laws of physics are generally symmetric under time inversion, whereas one is commonly aware of the passage of time from past to future. Whereas a mathematical description of a physical event is blind to the direction of time, human perception has absolutely no trouble in distinguishing time running backwards or forwards. One can look at a movie of a diver plunging into a swimming pool, which has been run backwards, and one is immediately aware that something is wrong with respect to time. This occurs because the time in the movie is not running forwards in the manner that we generally perceive it to run in the course of normal life. Here again I suggest we have a situation where physical theory can provide a reductive description of events in many respects but cannot recover the evolutionary element of time running forwards.[14]
With these views in mind one would have to agree with Lombardi and LaBarca’s
suggestion that the ontological reduction of chemistry in particular is not a
foregone conclusion and that more attention needs to be devoted to this issue.
Moreover, in spite of what I stated earlier concerning the reduction of
chemistry being almost complete, I support the notion that chemical phenomena
emerge from physical phenomena.
Notes
[1] Knight at least provides a qualified version of this claim, although he presents a chapter with the title "Chemistry a Reduced Science".
[2] Similar views regarding the reduction of chemistry to quantum mechanics have been expressed by Popper (1974) and Suppes (1984).
[3] Ambiguities are frequently discussed as paradoxes in the physics literature. Here the term ‘paradox’ will be resisted in order not to imply that they are of the same character as philosophical paradoxes such as Russell’s paradox of the liar for example.
[4] In recent years some articles have appeared in which it is claimed that electrons do have substructure (Maris 2000).
[5] The embrace of paradox, which is usually regarded as being somewhat alien in Western thought, is precisely what is often celebrated in Eastern philosophical worldviews. It is only in this very general sense, I suggest, that one should be willing to accept the possibility of connections between modern physics and Eastern philosophy. Modern physics demands that we take paradox seriously in the sense of a peaceful co-existence rather than something that must be cleared-up by future research. Perhaps one might embrace paradox in quantum physics as is done in Eastern philosophy instead of regarding it as an obstacle to deeper knowledge?
[6] With the possible exception of the work on quantum decoherence as a means of circumventing the collapse of the wave function issue (Tegmark & Wheeler, 2001).
[7] The present author must confess to also having made this mistake in an earlier paper where he and a co-author implied that the ontological reduction of chemistry was unproblematic (Scerri & McIntyre 1997).
[8] Incidentally, this is the same as Le Poidevin’s sense of ontological reduction but not quite the sense used by Lombardi and Labarca who allow theoretical considerations to come into ontological conclusions.
[9] The two problems are the vacuity problem and the symmetry problem. Physicalist claims that a perfected or eventual physics can explain all larger scale phenomena is vacuous since it is not clear what such a perfected physics might look like or simply because such a claim begs the questions of whether physics can indeed explain everything. The symmetry problem relates to the fact that it is not clear whether microscopic events fix macroscopic ones or vice versa. The intuition from the study of mental events is that mental acts determine lower levels whereas from the scientific perspective the intuition seems to be that microscopic components determine the behavior of macroscopic matter and perhaps even the behavior of sentient beings.
[10] My response to Le Poidevin’s wider claim that he has also shown that chemistry as a whole is ontologically reduced to physics is due to appear in the proceedings of the PSA for 2006 (Scerri, in press).
[11] I accept that some of these authors may be directing their comments at the claim that chemistry is ontologically reduced to chemistry but in any case my critique of their avoidance of the quantum mechanical details still stands.
[12] There follows an informal discussion of emergence with little or no connection to the formal debates that have taken place in recent philosophy of science (Kim 1999). I am also not intending emergence in the same sense as Broad as discussed earlier.
[13] For the present purposes it does not matter whether carbon atoms were literally formed by the addition of elementary particles to atoms of boron or whether the appropriate number of particles condensed together from scratch to form atoms of carbon.
[14] Of course the science of thermodynamics, and
especially the second law, does describe the time evolution of physical and
chemical processes. The problem has been to connect the thermodynamic arrow of
time with other laws of physics, which do not betray the directionality of time
(Albert 2000).
References
Albert, D.: 2000, Time and Chance, Cambridge MA: Harvard University Press.
Baggott, J.: 1992, The meaning of quantum theory: a guide for students of chemistry and physics, Oxford, New York: Oxford University Press.
Bensaude, B., Stengers, I.: 1996, A History of Chemistry, Cambridge MA: Harvard University Press.
Dirac, P.A.M.: 1929, ‘Quantum Mechanics of many-Electron Systems’, Proceedings of the Royal Society of London, A123, 714-733.
Dumas, J.B.: 1837, Leçons sur la philosophie chimique, Paris: M. Bineau (reprinted: Paris, 1878).
Gell-Mann, M.: 1994, ‘The Quark and the Jaguar: adventures in the simple and the complex’, New York: W.H. Freeman.
Hendry, R.F.: 1998, ‘Models and Approximations in Quantum Chemistry’, Poznan Studies in the Philosophy of Science and the Humanities, 63, 123-142.
Kim, J.: 1999, Making Sense of Emergence, Philosophical Studies, 95, 3-36.
Knight, D.: 1992, Ideas in Chemistry, A History of the Science, New Brumswick, NJ: Rutgers University Press.
Le Poidevin, R.: 2005, ‘Missing Elements and Missing Premises: A Combinatorial Argument for the Ontological Reduction of Chemistry’, British Journal for the Philosophy of Science, 56, 117-134.
Lombardi, O.; La Barca, M.: 2005, ‘The Ontological Autonomy of the Chemical World’, Foundations of Chemistry, 7, 125-148.
Maris H.J.: 2000, ‘On the Fission of Elementary Particles and the Evidence for Fractional Electrons in Liquid Helium’, Journal of Low Temperature Physics, 120, 173-204.
McLaughlin, B.: 1992, ‘The Rise and Fall of British Emergentism’, in: A. Beckerman, H. Flohr & J. Kim (eds.), Emergence or Reduction? Essays on the Prospect of a Non-Reductive Physicalism, Berlin: Walter de Gruyter, pp. 49-93.
Munowitz, M.: 2000, The Principles of Chemistry, New York: Norton.
Needham, P.: 1993, ‘Stuff’, Australasian Journal of Philosophy, 71, 270-290.
Popper, K.R.: 1974, ‘Scientific Reduction and the Essential Incompleteness of Science’, in: F.L. Ayala, T. Dobzhansky (eds.), Studies in the Philosophy of Science, Berkeley, CA: University of California Press, pp. 258-284.
Psarros, N.: 1997, ‘Critical Rationalism in the Test Tube?’, Journal for General Philosophy of Science, 28, 297-305.
Ramsey, J.L.: 1997, ‘Molecular Shape, Reduction, Explanation and Approximate Concepts’, Synthese, 111, 233-251.
Rothman, M.A.: 1992, The Science Gap: Dispelling the Myths and Understanding the Reality of Science, Buffalo, NY: Prometheus.
Scerri, E.R.: 1994, ‘Has Chemistry Been at Least Approximately Reduced to Quantum Mechanics?’, PSA 1, (East Lansing, MI: Philosophy of Science Association), 160-170.
Scerri, E.R.: 1998, ‘Popper’s Naturalized Approach to the Reduction of Chemistry’, International Studies in the Philosophy of Science, 12, 33-44.
Scerri, E.R.: 1999, ‘The Quantum Mechanical Explanation of the Periodic System (Author Reply)’, Journal of Chemical Education, 76, 1189.
Scerri, E.R.: 2004, ‘How ab initio is ab initio quantum chemistry’, Foundations of Chemistry, 6, 93-116.
Scerri, E.R.: 2007, The Periodic Table: Its Story and Its Significance, Oxford University Press, New York.
Scerri, E.R.: in press, ‘Reduction and Emergence in Chemistry – Two Recent Approaches’, Proceedings of the Philosophy of Science Association 2006.
Schummer. J.: 1997, ‘Towards a Philosophy of Chemistry’, Journal for General Philosophy of Science, 28, 307-336.
Suppes, P.: 1984, Probabilistic Metaphysics, Oxford: Blackwell.
Tegmark, M.; Wheeler, J.A.: 2001, ‘100 Years of Quantum Mysteries’, Scientific American, February, 68-75.
van Brakel, J.: 2000, Philosophy of Chemistry. Between the Manifest and the Scientific Image, Leuven, Leuven University Press.
Weininger, S.J.: 1984, ‘The Molecular Structure Conundrum: Can Classical Chemistry Be Reduced to Quantum Chemistry?’, Journal of Chemical Education, 61, 939-44.
Wilson, E.O.: 1998, Consilience, New York: Knopf.
Woody, A.I.: 2000, ‘Putting Quantum Mechanics to Work in Chemistry: The Power of Diagrammatic Representation’, Philosophy of Science (Proceedings), 67: S612-S627.
Woolley, R.G.: 1985, ‘The Molecular Structure Conundrum’, Journal of Chemical Education, 62, 1082-5.
UCLA, Department of Chemistry & Biochemistry, Los Angeles,
CA 90095, U.S.A.; scerri@chem.ucla.edu
Copyright © 2007 by HYLE and Eric R. Scerri